number of neutrons in chlorine|#17 : Baguio Chlorine is the 17th element in the periodic table and has a symbol of Cl and atomic number of 17. It has an atomic weight of 35.450 and a mass number of 35. Chlorine . 2. LeBlanc Hotel and Resort. First opened in January 2013, the LeBlanc Hotel and Resort is located at 3 Taktak Road, 191 Sen. L. Sumulong Memorial Circle, Brgy. Dela Paz, Antipolo City. It is near New Antipolo Public Market, Antipolo City Commercial Complex, and Robinsons Place Mall.
PH0 · Protons, Neutrons, Electrons for Chlorine (Cl, Cl–)
PH1 · How many neutrons are in an atom of chlorine?
PH2 · Chlorine (Cl)
PH3 · Chlorine (Cl)
PH4 · Chlorine
PH5 · Chemical Elements.com
PH6 · 2.4: Neutrons: Isotopes and Mass Number Calculations
PH7 · #17
APPLICATION IDEA 2nd method of using cove lighting – straight run coves in floating ceiling HYDRALUX™ IP67 with adhesive tape Photo: Florian Bouvet Fournier Field cuttable LED light line with minimum cross section size installed as a clas -
number of neutrons in chlorine*******The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron .
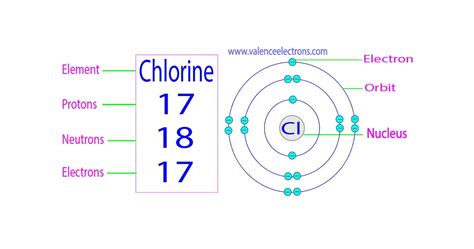
The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, . The number of neutrons in the nucleus of a Chlorine atom is 35 or 37, depending on the isotope. The number of protons is 17 and the number of electrons is .Chlorine is the 17th element in the periodic table and has a symbol of Cl and atomic number of 17. It has an atomic weight of 35.450 and a mass number of 35. Chlorine .Number of protons: 17 p + Number of neutrons: 18 n 0: Number of electrons: 17 e-Number of Neutrons: 18. Classification: Halogen. Crystal Structure: Orthorhombic. Density @ 293 K: 3.214 g/cm 3. Color: green. Atomic Structure. Isotopes. Facts. Date of Discovery: 1774. Discoverer: Carl .number of neutrons in chlorineChlorine: Symbol: Cl Atomic Number: 17: Atomic Mass: 35.453 atomic mass units: Number of Protons: 17: Number of Neutrons: 18: Number of Electrons: 17: Melting . The web page provides the answer to the question of how many neutrons are in an atom of chlorine, using the formula for the mass number of an atom. The answer is 18, which is the mass of chlorine .#17 The number of neutrons in the isotope can be calculated from its mass number, which is written as a superscript in a nuclear symbol. Mass Number = # of Protons + # of Neutrons 60 = 27 + # of Neutrons
Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) ( + 1) and a mass of 1 atomic mass unit (amu) ( amu), which is about 1.67 ×10−27 1.67 × 10 − 27 kilograms. Together with neutrons, they make up virtually all of the mass of an atom. Although all atoms of an element have the same number of protons, the atoms may differ in the number of neutrons they have (Table 1-2). These differing atoms of the same element are called isotopes. Four isotopes of helium (He) are shown in Figure 1-1. All atoms of chlorine (Cl) have 17 protons, but there are chlorine isotopes having 15 to .
X Research source. 5. Subtract the atomic number from the atomic mass. Since the vast majority of an atom’s mass is made up of its protons and neutrons, subtracting the number of .
Chlorine is a chemical element of the periodic table with chemical symbol Cl and atomic number 17 with an atomic weight of 35.446 u and is classed as a nonmetal. . Number of neutrons: 18 n 0: Number of electrons: 17 e-From Wikipedia, the free encyclopediaChlorine is a chemical element with symbol Cl and atomic number 17. It also has a .
number of neutrons in chlorine #17 Since we know that the mass of Chlorine is #35#, its atomic number is #17# and atomic number is the same as the proton it contains. Subtract the number of protons to the atomic mass . #35-17=18#
The atomic number of iodine (53) tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74).Name: Chlorine: Symbol: Cl Atomic Number: 17: Atomic Mass: 35.453 atomic mass units: Number of Protons: 17: Number of Neutrons: 18: Number of Electrons: 17: Melting Point
Mass Number = # of Protons + # of Neutrons. Mass Number = 1 + 2. Therefore, this particular atom of hydrogen will have a mass number of 3. Note that the mass number calculated in Example 2.4.1 2.4. 1 does not match the number underneath the elemental symbol and name for hydrogen on the periodic table. Because chlorine has an atomic number of 17, chlorine has 17 protons, 18, neutrons, and 17 electrons; 118 neutrons; 6 protons, 8 neutrons, and 6 electrons; Z=3, 3 protons, 2 electrons; The mass number represents the average mass of all of the isotopes of that particular element.
Notes (related to the columns): 1 - name of the nuclide, isotope. 2 - E: isotope symbol with mass number (superscript; number of nucleons) and Atomic number (subscript; number of protons). 3 - N: number of neutrons. 4 - relative atomic mass of the Chlorine isotope (isotopic mass including electrons) and the mass of the atomic nucleus in square .

Atoms of the same element with different numbers of neutrons are called isotopes close isotope Atoms of an element with . the relative atomic mass of chlorine is 35.5 rather than a whole number .Protons and Neutrons in Sulfur. Sulfur is a chemical element with atomic number 16 which means there are 16 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is . For many other elements, however, more than one isotope may exist in substantial quantities. Chlorine (atomic number 17) is yellowish-green toxic gas. About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a .
Name: Chlorine: Symbol: Cl Atomic Number: 17: Atomic Mass: 35.453 atomic mass units: Number of Protons: 17: Number of Neutrons: 18: Number of Electrons: 17: Melting Point The number of neutrons in chlorine can be obtained by subtracting the atomic number from its atomic mass. The atomic mass of chlorine is 35.45 u (which you can round it to 34). So from this atomic mass (i.e 34), you have to subtract its atomic number (i.e 17). For any atom number of neutrons present in the nucleus is given by the following formula: Number of neutrons = Atomic mass (rounding it up to the nearest whole number) – Number of protons. As mentioned in the chlorine box drawn above the atomic mass of the chlorine atom is 35.353. After rounding it up to the nearest whole number .
Give the symbol of each isotope with the mass number as the superscript and the number of protons as the subscript, both written to the left of the symbol of the element. Solution: A The element with 82 protons (atomic number of 82) is lead: Pb. B For the first isotope, A = 82 protons + 124 neutrons = 206.Name: Chlorine Symbol: Cl Atomic Number: 17 Atomic Mass: 35.4527 amu Melting Point:-100.98 °C (172.17 K, -149.764 °F) Boiling Point:-34.6 °C (238.55 K, -30.279997 °F) Number of Protons/Electrons: 17 Number of Neutrons: 18 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.214 g/cm 3 Color: green Atomic Structure
Leftovers garante a Empoleon uma forma passiva de recovery, aumentando sua longevidade no decorrer da partida. Torrent é a ability mais útil de Empoleon, visto que potencializa Scald quando seu HP está baixo. Dicas de Uso. Empoleon deve ser usado como uma switch-in a ameaças como Latias, Primarina, Mega Pidgeot e Mega .
number of neutrons in chlorine|#17